Blood Collection & Processing Consumables
Blood Bank Solutions
Comprehensive systems designed to support safe blood collection, processing, storage, and transfusion management.
WEGO’s Blood Bank Solutions deliver a fully integrated system that supports the complete lifecycle of blood collection, processing, storage, and transfusion across hospital blood banks, regional blood centers, and national donation programs. Built on decades of manufacturing expertise in sterile medical consumables, our solution combines high-quality blood bags, advanced leukocyte reduction technology, and validated temperature-controlled transport equipment to ensure that every unit of donated blood is handled with maximum safety and efficiency.
From donor screening and whole-blood collection to component separation and final transfusion, each element of the WEGO system is designed to maintain a closed, sterile, and traceable workflow. The blood bag portfolio (single, double, triple, and quadruple systems) enables precise preparation of red cells, plasma, platelets, and cryoprecipitate, supporting diverse clinical needs and optimizing the utilization of each donation. Integrated leukocyte reduction solutions help minimize febrile reactions, alloimmunization risks, and leukocyte-related pathogen transmission, meeting global transfusion standards such as AABB and EDQM.
To safeguard blood stability during distribution, WEGO’s insulated transport boxes deliver validated cold-chain performance, maintaining required temperatures across hospital networks, mobile donation units, and inter-facility logistics. Together, these technologies form a robust, interoperable ecosystem that enhances operational consistency, strengthens transfusion safety, and supports scalable blood-service infrastructure for both routine operations and emergency response scenarios.
Whether your institution manages daily donor collections, handles regional supply coordination, or prepares specialized blood components for critical care, WEGO provides a reliable, end-to-end solution that aligns with modern clinical, regulatory, and workflow requirements—empowering healthcare providers to deliver safe and effective transfusion therapy with confidence.

WEGO triple bag for blood collection is designed for the separation into three distinct components from human whole blood, such as red blood cells, plasma, and platelets. The triple SAGM blood bag system includes one primary bag with anticoagulant CPD solution USP, SAGM solution in a another bag, and one empty satellite bag. WEGO Medical manufactures ideal triple collection bags for blood banks to maximize the utility of a single donation by providing multiple components for different therapeutic uses.
WEGO quadruple blood collection bag system is a more complex setup consisting of one primary bag and three separate satellite bags connected by tubing. It allows for the simultaneous collection of whole blood and the separation into four different blood components. The quadruple SAGM blood bag system includes one primary bag with anticoagulant CPD solution USP, SAGM solution in a second bag, and two empty satellite bags. The system is made from medical-grade PVC materials using plastic blow molding technology, featuring convenient hanger slits and holes. It includes a 16G sharp-pointed Japanese needle with a siliconized ultra-thin wall, with a 17G needle also available. It is equipped with standard donor tubing and has a code number printed on the tube surface.

Blood Bank Solutions
Blood centers and hospital blood banks form the backbone of national transfusion services, ensuring the safe collection, processing, storage, and distribution of blood and its components. Their operational quality directly determines the clinical availability, safety, and therapeutic effectiveness of transfusion therapy. As demand for precise component therapy, centralized screening, and emergency preparedness continues to rise, blood services require integrated solutions that support reliability, standardization, and regulatory compliance across the entire workflow.
Modern blood centers must manage complex processes including donor management, whole-blood collection, leukocyte reduction, component separation, infectious disease screening, labeling, traceability, storage, and inter-facility distribution. Each step relies on closed-system consumables, validated cold-chain infrastructure, and sterility-controlled environments to prevent contamination, maintain component viability, and ensure adherence to AABB, EDQM, and WHO transfusion standards. In hospital blood banks, rapid accessibility, product accuracy, and emergency-readiness are equally critical, with emphasis on compatibility testing, inventory optimization, and secure issue procedures.
To support these operational demands, WEGO delivers an end-to-end platform designed specifically for blood-service institutions—enabling consistent product quality, reducing process variation, and enhancing overall transfusion safety. Through interoperable blood bag systems, leukocyte reduction technologies, and validated temperature-controlled transport solutions, WEGO helps blood centers strengthen routine operations, expand donor programs, and ensure stable blood supply even under high-pressure clinical or emergency scenarios.
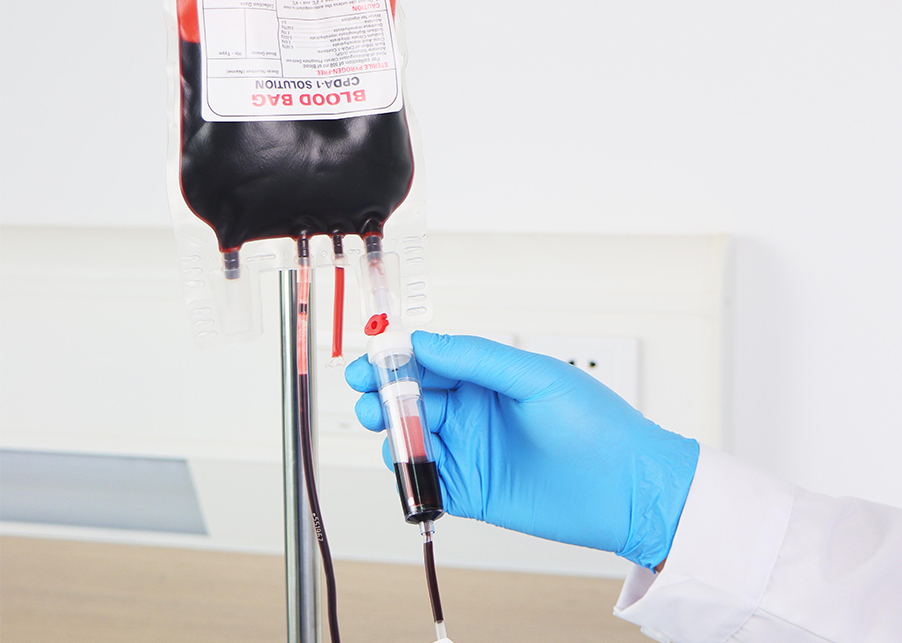
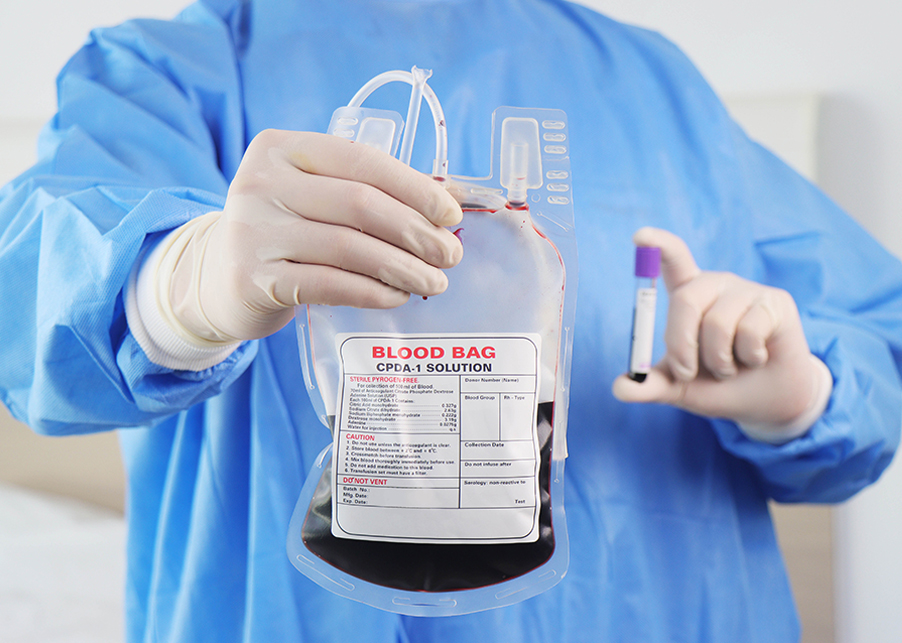

Blood collection bags can be classified by material technology, system design and additives, ensuring safe whole blood collection, efficient component preparation, and contamination-free transfusion workflows.
According to Production Material
Calendared Film Blood Bags :
Manufactured through a calendaring process that produces highly uniform, clear, and mechanically strengthened PVC film. This material offers superior puncture resistance, low permeability, and excellent durability during centrifugation, making it suitable for high-precision blood component separation.
Extruded Film Blood Bags :
Made from conventional medical-grade PVC film. These bags provide stable sealing, reliable flexibility, and good compatibility with routine collection, mixing, and storage, making them widely used in standard blood banking operations.


According to System Design
Single Blood Bag:
Designed for whole blood collection, storage, and direct transfusion. It supports basic donation programs and routine clinical transfusion needs.
Double Blood Bag:
Allows whole blood to be separated into plasma and red blood cells after centrifugation. Commonly used in hospitals and blood centers to optimize blood resource utilization.
Triple Blood Bag:
Enables the preparation of red cells, plasma, and platelet concentrate from one donation. Essential for component therapy in surgery, oncology, and emergency medicine.
Quadruple Blood Bag:
Supports the complete separation into four components, including cryoprecipitate, enabling maximum therapeutic output from a single unit of blood. Widely used in high-volume blood centers.
According to Blood Bag Additives
CPDA-1 (Citrate Phosphate Dextrose Adenine):
A widely used additive solution that maintains red blood cell integrity and metabolic activity during storage. It consists of citrate (anticoagulant), phosphate (buffer), dextrose (energy source), and adenine (preserves ATP levels). CPDA is ideal for storing red blood cells for up to 21 days, ensuring optimal viability for transfusion.
SAGM (Saline-Adenine-Glucose-Mannitol):
An alternative additive solution, SAGM is designed to extend the storage of red blood cells beyond the limits of CPDA. This solution contains saline (buffer), adenine (for ATP preservation), glucose (as an energy source), and mannitol (which prevents red cell dehydration). SAGM-stored red blood cells can remain viable for up to 42 days, making it a preferred choice for long-term storage in large blood banks and hospitals.


Unmatched Sterility & Safety
WEGO blood bags are engineered with a closed, sterile system, significantly minimizing contamination risks throughout the entire blood collection, processing, and transfusion cycle. Our advanced design prevents microbial contamination, air exposure, and leakage, ensuring that each blood bag provides the highest level of safety for patients and healthcare professionals. With global compliance to industry-leading standards such as ISO 13485 and CE, WEGO blood bags ensure that you are using the safest solution available for blood collection and transfusion.
Premium Materials
As the NO.1 choice for blood collection bags in China, We uses only medical-grade PVC that is biocompatible, low in leachability, and highly resistant to temperature variations, ensuring that blood components remain in optimal condition for storage and transfusion. Our materials are rigorously tested for durability, ensuring long-term stability and reliability, from storage to transfusion. The transparent design of WEGO blood bags allows for easy visual inspection, guaranteeing clear monitoring of blood quality and early detection of any abnormalities during storage.

Flexible Solutions
WEGO blood bags offer multi-component separation solutions, available in single, double, triple, and quadruple configurations, making them ideal for diverse clinical scenarios, from routine blood transfusions to complex component therapies. With CPDA-1 and SAGM preservatives, WEGO blood bags extend red blood cell storage up to 42 days, offering maximum flexibility in blood resource management. Whether you are a high-volume blood bank or a regional transfusion center, WEGO ensures that you have the most efficient, safe, and reliable system to support your blood collection and processing needs.

Q1. Can WEGO customize blood bags for specific country requirements?
A: Yes. Customization options include:
tubing length
needle size
anticoagulant volume
labeling and language requirements
Q2. How strong is WEGO’s manufacturing capacity for blood bags?
A: WEGO operates multiple large-scale, highly automated production lines dedicated to blood collection bags, supporting high-volume and continuous manufacturing. This enables us to provide stable, long-term supply to national blood centers, hospitals, and global distributors—even during peak demand or emergency periods.
Q3. How does WEGO ensure the sterility and safety of every blood bag?
A: All blood bags undergo 100% sterilization using validated processes (such as steam or gamma sterilization). Each batch passes rigorous testing, including leak testing, tensile strength, biocompatibility, and solution stability. WEGO follows GMP and ISO13485 quality systems to guarantee product sterility and clinical safety.
Q4. Can WEGO support large-scale procurement projects or national tenders?
A: Yes. With strong production capabilities and a mature supply chain, WEGO consistently supports large-volume government tenders, emergency medical reserves, and long-term hospital procurement without risking stockouts. Our flexible manufacturing system allows rapid capacity expansion when needed.
Q5. Does WEGO offer customized blood bag configurations for different markets?
A: Yes. Our engineering and production teams can adapt bag volumes, anticoagulants (CPDA-1 / SAGM), tubing lengths, labels, connectors, and multi-bag configurations to meet national standards, regulatory requirements, and hospital workflows.








